There are many different fuel cell types. The differences between them are the reactions at the electrodes and the electrolyte used.
Acid Electrolyte fuel cell
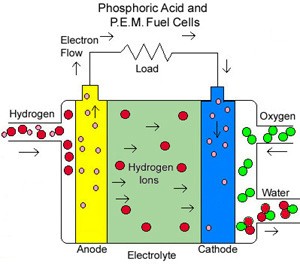
The most common type of fuel cell is the acid electrolyte, seen in figure below. At the anode the following reaction takes place
Hydrogen gas ionizes into hydrogen ions and electrons, equation. The electrons released from the reaction flow through an external load to the cathode, creating a current. The H+ ions pass through the electrolyte which is possible because an acid is a fluid with mobile H+ ions. At the cathode the H+ reacts with oxygen and the electrons forming water, equation
The electrons are not allowed to pass through the electrolyte because then no current would flow in the external circuit.
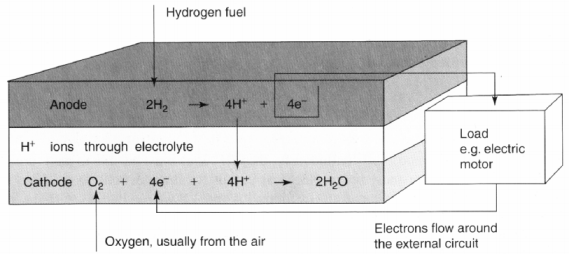
Electrode reactions and electrical flow for an acid electrolyte fuel cell. The electrons flow from the anode to the cathode via an external circuit.
Alkaline Electrolyte fuel cell
In the Alkaline electrolyte the reaction is similar to the acid electrolyte but the reactions at each electrode are different. In this case the mobile ions are hydroxyl ions (OH-). The hydrogen fuel reacts with OH- at the anode producing water and releasing electrical energy. At the cathode side the electrons released from the anode reacts with oxygen and water creating new OH- ions, equation below. Although water is consumed at the cathode, twice the amount is produced at the anode
As for the acid electrolyte there must be an external load so that the electrons can flow from the anode to the cathode, as in figure below
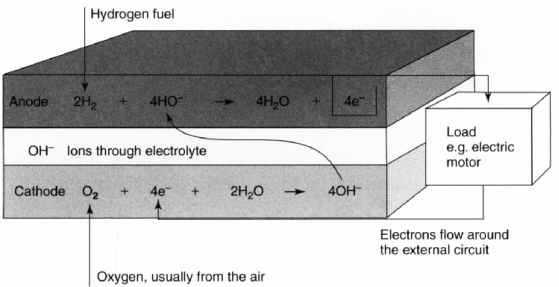
Electrode reactions and electrical flow for an alkaline electrolyte fuel cell.
Both the acid electrolyte and the alkaline electrolyte twice as many moles of hydrogen as of oxygen are needed in the reaction.
Proton exchange membrane fuel cell
The Proton Exchange Membrane (PEM) fuel cell is the most useful at present time. A solid polymer, in which protons (H+) are mobile, is used as electrolyte. The fact that the electrolyte is solid and immobile makes this cell very simple. The working temperature of these cells is quite low, around 30-100°C, which gives a problem of slow reaction rates but at the same time the startup is fast. The problem of slow reaction rates is solved by the use of more effective electrodes and catalysts such as platinum. Another drawback is that the membrane is fragile and breaks easily. The field of application is essentially in vehicles, portable applications and low power CHP (Combined Heat and Power) systems.
Direct-methanol fuel cells or DMFCs – They are a subcategory of proton-exchange fuel cells in which methanol is used as the fuel. Their main advantage is the ease of transport of methanol, energy dense yet reasonably stable liquid at all environmental conditions. Efficiency is quite low for these cells, so they are targeted especially to portable applications, where energy and power density are more important than efficiency.
Reformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems – They are a subcategory of proton-exchange fuel cells where, the fuel, methanol(CH3OH), is reformed, before being fed into the fuel cell. RMFC systems offer advantages over direct methanol fuel cell (DMFC) systems including higher efficiency, smaller cell stacks, no water management, better operation at low temperatures, and storage at sub-zero temperatures because methanol is a liquid from -97.0 °C to 64.7 °C (-142.6 °F to 148.5 °F). The tradeoff is that RMFC systems operate at hotter temperatures and therefore need more advanced heat management and insulation. The waste products with these types of fuel cells are carbon dioxide and water
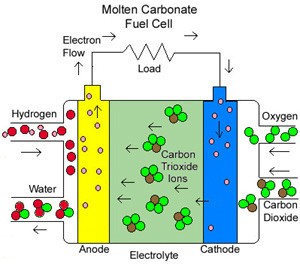
Molten Carbonate fuel cells (MCFC)
They use high-temperature compounds of salt (like sodium or magnesium) carbonates (chemically, CO3) as the electrolyte. Efficiency ranges from 60 to 80 percent, and operating temperature is about 650 degrees C (1,200 degrees F). Units with output up to 2 megawatts (MW) have been constructed, and designs exist for units up to 100 MW. The high temperature limits damage from carbon monoxide “poisoning” of the cell and waste heat can be recycled to make additional electricity. Their nickel electrode-catalysts are inexpensive compared to the platinum used in other cells. But the high temperature also limits the materials and safe uses of MCFCs–they would probably be too hot for home use. Also, carbonate ions from the electrolyte are used up in the reactions, making it necessary to inject carbon dioxide to compensate.
Phosphoric Acid fuel cells (PAFC)
They use phosphoric acid as the electrolyte. Efficiency ranges from 40 to 80 percent, and operating temperature is between 150 to 200 degrees C (about 300 to 400 degrees F). Existing phosphoric acid cells have outputs up to 200 kW, and 11 MW units have been tested. PAFCs tolerate a carbon monoxide concentration of about 1.5 percent, which broadens the choice of fuels they can use. If gasoline is used, the sulfur must be removed. Platinum electrode-catalysts are needed, and internal parts must be able to withstand the corrosive acid.
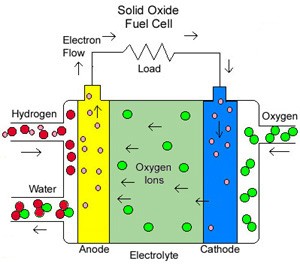
Solid Oxide fuel cells (SOFC)
They use a hard, ceramic compound of metal (like calcium or zirconium) oxides (chemically, O2) as electrolyte. Efficiency is about 60 percent, and operating temperatures are about 1,000 degrees C (about 1,800 degrees F). Cells output is up to 100 kW. At such high temperatures a reformer is not required to extract hydrogen from the fuel, and waste heat can be recycled to make additional electricity. However, the high temperature limits applications of SOFC units and they tend to be rather large. While solid electrolytes cannot leak, they can crack.