Over 85% of the energy used in the world is non renewable. Most developed nations rely on non-renewable energy sources such as fossil fuels (coal and oil) and nuclear power to meet the requirements of the citizens, in particular and the nation, in general. These sources are called non-renewable because they cannot be renewed or regenerated quickly enough to keep pace with their use.
Industrialized societies depend on non-renewable energy sources: Fossil fuels are the most commonly used types of non-renewable energy. They were formed when incompletely decomposed plant and animal matter was buried in the earth’s crust and transformed into carbon-rich material that is useable as fuel. This process occurred over millions of years i.e. a geological time frame. The three main types of fossil fuels are coal, oil, and natural gas. Two other less-used sources of fossil fuels are oil shales and tar sands.
COAL
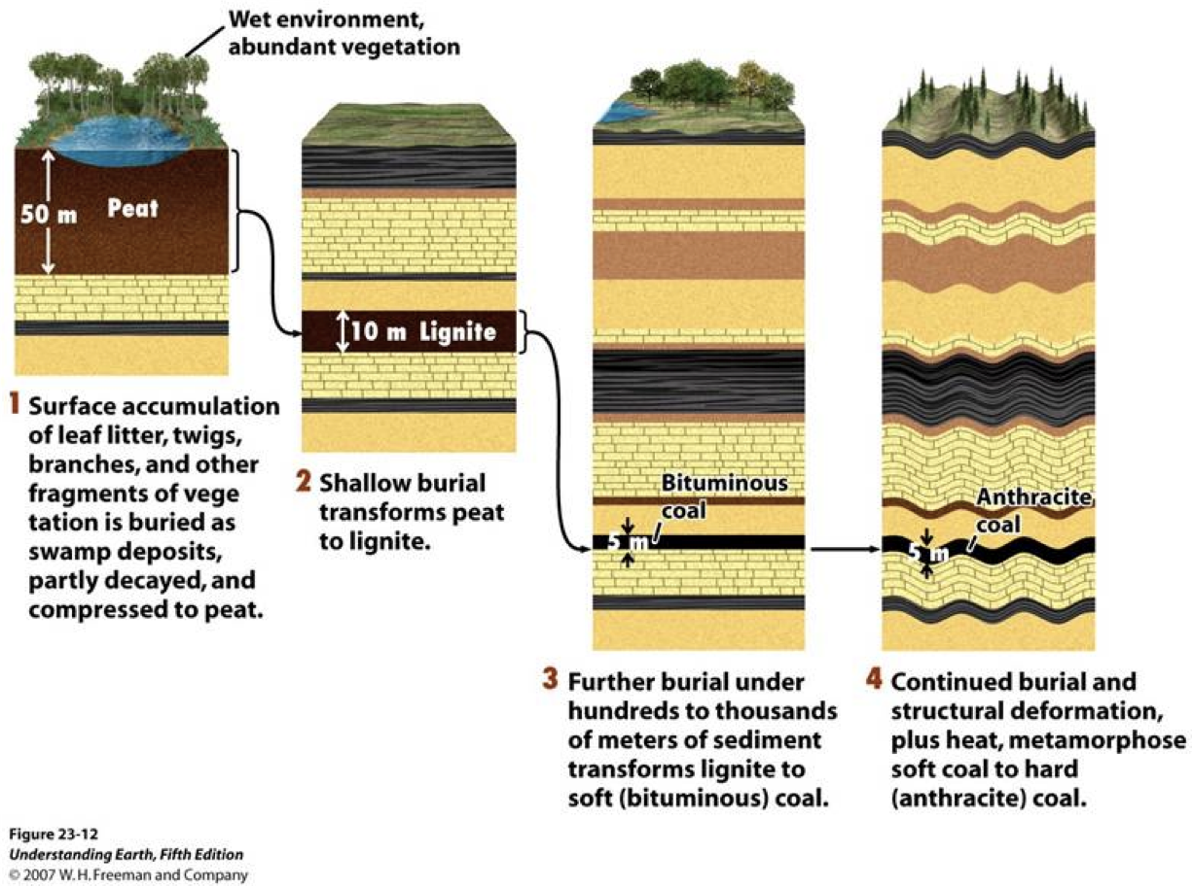
Coal is the most abundant fossil fuel in the world with an estimated reserve of one trillion metric tons. Most of the world’s coal reserves exist in Eastern Europe and Asia, but the United States also has considerable reserves. Coal formed slowly over millions of years from the buried remains of ancient swamp plants. During the formation of coal, carbonaceous matter was first compressed into a spongy material called “peat,” which is about 90% water. As the peat became more deeply buried, the increased pressure and temperature turned it into coal.
Different types of coal resulted from differences in the pressure and temperature that prevailed during formation. The softest coal (about 50% carbon), which also has the lowest energy output, is called lignite. Lignite has the highest water content (about 50%) and relatively low amounts of smog-causing sulfur. With increasing temperature and pressure, lignite is transformed into bituminous coal (about 85% carbon and 3% water). Anthracite (almost 100% carbon) is the hardest coal and also produces the greatest energy when burned. Less than 1% of the coal found in the United States is anthracite. Most of the coal found in the United States is bituminous.
Unfortunately, bituminous coal has the highest sulfur content of all the coal types. When the coal is burned, the pollutant sulfur dioxide is released into the atmosphere
The Process of Coal Formation
Coal has many important uses worldwide. The most significant uses of coal are in electricity generation, steel production, cement manufacturing and as a liquid fuel. Around 6.6 billion tonnes of hard coal were used worldwide last year and 1 billion tonnes of brown coal.
Since 2000, global coal consumption has grown faster than any other fuel. The five largest coal users – China, USA, India, Russia and Japan – account for 76% of total global coal use.
Different types of coal have diverse uses. Steam coal – also known as thermal coal – is mainly utilized in power generation. Coking coal – also known as metallurgical coal – is mainly used in steel production.
The biggest market for coal is Asia, which currently accounts for over 67% of global coal consumption; although China is liable for a significant proportion of this. Many countries do not have natural energy resources sufficient to cover their energy needs, and therefore need to import energy to help meet their requirements. Japan, Chinese Taipei and Korea, for example, import significant quantities of steam coal for electricity generation and coking coal for steel production. If the exports are less than the imports, this leads to a fall in the net exports and therefore in the volume of trade of these countries. This undoubtedly will have a detrimental effect on the coal industry of these economies. If, however, the volume of trade in the other industries is higher, the economies will perform well in the world market.
Other important users of coal include alumina refineries, paper manufacturers, and the chemical and pharmaceutical industries. Several chemical products can be produced from the by-products of coal. Refined coal tar is used in the manufacture of chemicals, such as creosote oil, naphthalene, phenol, and benzene. Ammonia gas recovered from coke ovens is used to manufacture ammonia salts, nitric acid and agricultural fertilisers.
Thousands of different products have coal or coal by-products as components: soap, aspirins, solvents, dyes, plastics and fibres, such as rayon and nylon. Coal is also an indispensable component in the production of specialist products:
Activated carbon: used in filters for water and air purification and in kidney dialysis machines.
Carbon fibre: an extremely strong but light weight reinforcement material used in construction, mountain bikes and tennis rackets.
Silicon metal: used to produce silicones and silanes, which are in turn used to make lubricants, water repellents, resins, cosmetics, hair shampoos and toothpastes.
This insinuates that, in the absence of coals, the performance and growth of innumerable economies in the world would have been considerably lower.
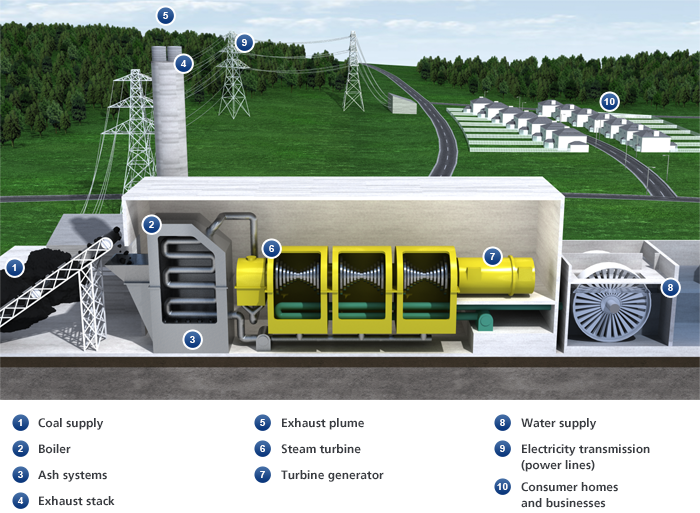
Coal Electricity Generation
A coal power station turns the chemical energy in coal into electrical energy that can be used in homes and businesses.
First the coal (1) is ground to a fine powder and blown into the boiler (2), where it is burned, converting its chemical energy into heat energy. Grinding the coal into powder increases its surface area, which helps it to burn faster and hotter, producing as much heat and as little waste as possible.
As well as heat, burning coal produces ash and exhaust gases. The ash falls to the bottom of the boiler and is removed by the ash systems (3). It is usually then sold to the building industry and used as an ingredient in various building materials, like concrete.
The gases enter the exhaust stack (4), which contains equipment that filters out any dust and ash, before venting into the atmosphere. The exhaust stacks of coal power stations are built tall so that the exhaust plume (5) can disperse before it touches the ground. This ensures that it does not affect the quality of the air around the station.
Burning the coal heats water in pipes coiled around the boiler, turning it into steam. The hot steam expands in the pipes, so when it emerges it is under high pressure. The pressure drives the steam over the blades of the steam turbine (6), causing it to spin, converting the heat energy released in the boiler into mechanical energy.
A shaft connects the steam turbine to the turbine generator (7), so when the turbine spins, so does the generator. The generator uses an electromagnetic field to convert this mechanical energy into electrical energy.
After passing through the turbine, the steam comes into contact with pipes full of cold water. In coastal stations this water is pumped straight from the sea (8). The cold pipes cool the steam so that it condenses back into water. It is then piped back to the boiler, where it can be heated up again, turn into steam again, and keep the turbine turning.
Finally, a transformer converts the electrical energy from the generator to a high voltage. The national grid uses high voltages to transmit electricity efficiently through the power lines (9) to the homes and businesses that need it (10). Here, other transformers reduce the voltage back down to a usable level.
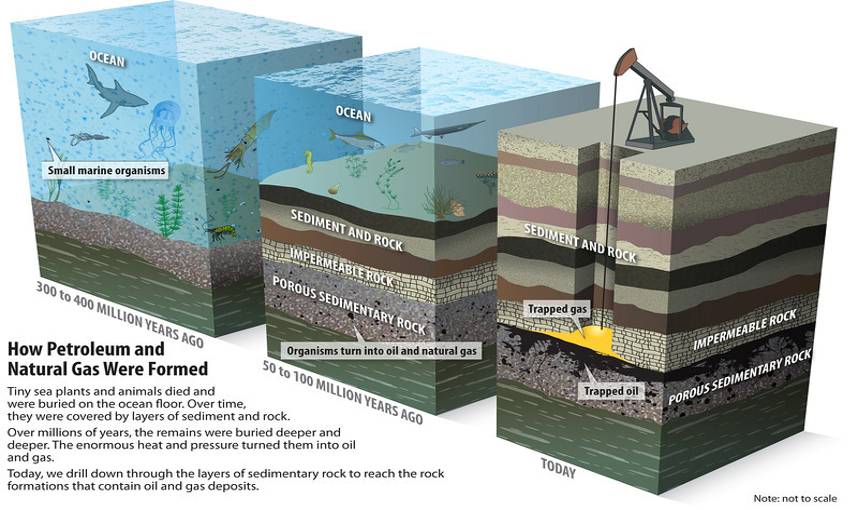
OIL
Crude oil or liquid petroleum, is a fossil fuel that is refined into many different energy products (e.g., gasoline, diesel fuel, jet fuel, heating oil). Oil forms underground in rock such as shale, which is rich in organic materials. After the oil forms, it moves upwards into porous reservoir rock such as sandstone or limestone, where it is trapped by an overlying impermeable cap rock. Wells are drilled into these oil reservoirs to remove the gas and oil. Over 70 percent of oil fields are found near tectonic plate boundaries, because the conditions there are conducive to oil formation.
Oil recovery may or may not have several stages. The primary stage involves pumping oil from reservoirs under the normal reservoir pressure. About 25 percent of the oil in a reservoir can be removed during this stage. The secondary recovery stage involves injecting hot water into the reservoir around the well. This water forces the remaining oil toward the area of the well from which it can be recovered. Sometimes a tertiary method of recovery is used in order to remove as much oil as possible. This involves pumping steam, carbon dioxide gas or nitrogen gas into the reservoir to force the remaining oil toward the well. Tertiary recovery is very expensive and can cost up to half of the value of oil removed. Economists would conduct a cost benefit analysis before this stage to ascertain whether the aggregate benefits of this tertiary recovery are more than the costs involved. Aggregate benefits imply the benefits to society. Carbon dioxide used in this method remains sequestered in the deep reservoir, thus mitigating its potential greenhouse effect on the atmosphere. The refining process required to convert crude oil into useable hydrocarbon compounds involves boiling the crude and separating the gases in a process known as fractional distillation. Besides its use as a source of energy, oil also provides base material for plastics, provides asphalt for roads and is a source of industrial chemicals. Over 50 percent of the world’s oil is found in the Middle East; ample additional reserves occur in North America. Most known oil reserves are already being exploited, and oil is being used at a rate that exceeds the rate of discovery of new sources. If the consumption rate continues to increase and no significant new sources are found, oil supplies may be exhausted in another 30 years or so. This is a matter of grave concern indeed. Despite its limited supply, oil is a relatively inexpensive fuel source. This is a surprising occurance, keeping the laws of Economics in mind. A plausible explanation could be that oil is a necessary good with an inelastic supply curve. Oil is a preferred fuel source over coal.
An equivalent amount of oil produces more kilowatts of energy than coal. It also burns cleaner, producing about 50 percent less sulfur dioxide. Environmental problems, also known as “economic bads” or “externalities” are an undeniable part of exploiting any natural resource. If the quantity of these externalities is more than the assimilative capacity of the environment, it will result in pollution/degradation of resources. This, in turn, will have adverse and severe implications for the health of all living beings and the well being of the nation . The burning of oil releases atmospheric pollutants such as sulfur dioxide, nitrogen oxides, carbon dioxide and carbon monoxide. These gases are smog-precursors that pollute the air and greenhouse gases that contribute to global warming. Another environmental issue associated with the use of oil is the impact of oil drilling. Substantial oil reserves lie under the ocean. Oil spill accidents involving drilling platforms kill marine organisms and birds. Some reserves such as those in northern Alaska occur in wilderness areas. The building of roads, structures and pipelines to support oil recovery operations can severely impact the wildlife in those natural areas.
Natural Gas
Natural gas production is often a by-product of oil recovery, as the two commonly share underground reservoirs. Natural gas is a mixture of gases, the most common being methane (CH4). It also contains some ethane (C2H5), propane (C3H8), and butane (C4H10). Natural gas is usually not contaminated with sulfur and is therefore the cleanest burning fossil fuel. After recovery, propane and butane are removed from the natural gas and made into liquefied petroleum gas (LPG). LPG is shipped in special pressurized tanks as a fuel source for areas not directly served by natural gas pipelines (e.g., rural communities). The remaining natural gas is further refined to remove impurities and water vapor, and then transported in pressurized pipelines. The United States has over 300,000 miles of natural gas pipelines. Natural gas is highly flammable and is odorless. The characteristic smell associated with natural gas is actually that of minute quantities of a smelly sulfur compound (ethyl mercaptan) which is added during refining to warn consumers of gas leaks. The use of natural gas is growing rapidly. Besides being a clean burning fuel source, natural gas is easy and inexpensive to transport once pipelines are in place. In developed countries, natural gas is used primarily for heating, cooking, and powering vehicles. It is also used in a process for making ammonia fertilizer. The current estimate of natural gas reserves is about 100 million metric tons. At current usage levels, this supply will last an estimated 100 years. Most of the world’s natural gas reserves are found in Eastern Europe and the Middle East.
Oil Shale and Tar Sands
Oil shale and tar sands are the least utilized fossil fuel sources. Oil shale is sedimentary rock with very fine pores that contain kerogen, a carbon-based, waxy substance. If shale is heated to 490º C, the kerogen vaporizes and can then be condensed as shale oil, a thick viscous liquid. This shale oil is generally further refined into usable oil products. Production of shale oil requires large amounts of energy for mining and processing the shale. Indeed about a half barrel of oil is required to extract every barrel of shale oil. Oil shale is plentiful, with estimated reserves totaling 3 trillion barrels of recoverable shale oil. These reserves alone could satisfy the world’s oil needs for about 100 years. Environmental problems associated with oil shale recovery include: large amounts of water needed for processing, disposal of toxic waste water, and disruption of large areas of surface lands. Tar sand is a type of sedimentary rock that is impregnated with a very thick crude oil. This thick crude does not flow easily and thus normal oil recovery methods cannot be used to mine it. If tar sands are near the surface, they can be mined directly. In order to extract the oil from deep-seated tar sands, however, steam must be injected into the reservoir to make the oil flow better and push it toward the recovery well. The energy cost for producing a barrel of tar sand is similar to that for oil shale. The largest tar-sand deposit in the world is in Canada and contains enough material (about 500 billion barrels) to supply the world with oil for about 15 years. However, because of environmental concerns and high production costs these tar sand fields are not being fully utilized.
Nuclear Power
In most electric power plants, water is heated and converted into steam, which drives a turbine-generator to produce electricity. Fossil-fueled power plants produce heat by burning coal, oil, or natural gas. In a nuclear power plant, the fission of uranium atoms in the reactor provides the heat to produce steam for generating electricity. Several commercial reactor designs are currently in use in the United States. The most widely used design consists of a heavy steel pressure vessel surrounding a reactor core. The reactor core contains the uranium fuel, which is formed into cylindrical ceramic pellets and sealed in long metal tubes called fuel rods.
Thousands of fuel rods form the reactor core. Heat is produced in a nuclear reactor when neutrons strike uranium atoms, causing them to split in a continuous chain reaction. Control rods, which are made of a material such as boron that absorbs neutrons, are placed among the fuel assemblies. When the neutron-absorbing control rods are pulled out of the core, more neutrons become available for fission and the chain reaction speeds up, producing more heat. When they are inserted into the core, fewer neutrons are available for fission, and the chain reaction slows or stops, reducing the heat generated. Heat is removed from the reactor core area by water flowing through it in a closed pressurized loop. The heat is transferred to a second water loop through a heat exchanger. The water also serves to slow down, or “moderate” the neutrons which is necessary for sustaining the fission reactions. The second loop is kept at a lower pressure, allowing the water to boil and create steam, which is used to power the turbine-generator and produce electricity.
Originally, nuclear energy was expected to be a clean and cheap source of energy. Nuclear fission does not produce atmospheric pollution or greenhouse gases and it proponents expected that nuclear energy would be cheaper and last longer than fossil fuels. Unfortunately, because of construction cost overruns, poor management, and numerous regulations, nuclear power ended up being much more expensive than predicted. The nuclear accidents at Three Mile Island in Pennsylvania and the Chernobyl Nuclear Plant in the Ukraine raised concerns about the safety of nuclear power. Furthermore, the problem of safely disposing spent nuclear fuel remains unresolved. The United States has not built a new nuclear facility in over twenty years, but with continued energy crises across the country that situation may change.
Nuclear power is the use of nuclear reactors to release nuclear energy, and thereby produce electricity. The term includes nuclear fission, nuclear decay and nuclear fusion. Presently, the nuclear fission of elements in the actinide series of the periodic table produce the vast majority of nuclear energy in the direct service of humankind, with nuclear decay processes, primarily in the form of geothermal energy, and radioisotope thermoelectric generators, in niche uses making up the rest. Nuclear (fission) power stations, excluding the contribution from naval nuclear fission reactors, provided 13% of the world’s electricity in 2012. The share of the world’s primary energy supply, which refers to the heat production without the conversion efficiency of about 33 %, was about 5.7%.Its share of the global final energy consumption (actually useful energy, i.e. electric power) is below 2.5 %.
In 2013, the IAEA report that there are 437 operational nuclear power reactors, in 31 countries, although not every reactor is producing electricity. In addition, there are approximately 140 naval vessels using nuclear propulsion in operation, powered by some 180 reactors. As of 2013, attaining a net energy gain from sustained nuclear fusion reactions, excluding natural fusion power sources such as the Sun, remains an ongoing area of international physics and engineering research. More than 60 years after the first attempts, commercial fusion power production remains unlikely before 2050.
There is an ongoing debate about nuclear power. Proponents, such as the World Nuclear Association, the IAEA and Environmentalists for Nuclear Energy contend that nuclear power is a safe, sustainable energy source that reduces carbon emissions. Opponents, such as Greenpeace International and NIRS, contend that nuclear power poses many threats to people and the environment.
Nuclear power plant accidents include the Chernobyl disaster (1986), Fukushima Daiichi nuclear disaster (2011), and the Three Mile Island accident (1979). There have also been some nuclear submarine accidents. In terms of lives lost per unit of energy generated, analysis has determined that nuclear power has caused less fatalities per unit of energy generated than the other major sources of energy generation. Energy production from coal, petroleum, natural gas and hydropower has caused a greater number of fatalities per unit of energy generated due to air pollution and energy accident effects. However, the economic costs of nuclear power accidents are high, and meltdowns can render areas uninhabitable for very long periods. The human costs of evacuations of affected populations and lost livelihoods are also significant.
Along with other sustainable energy sources, nuclear power is a low carbon power generation method of producing electricity, with an analysis of the literature on its total life cycle emission intensity finding that it is similar to other renewable sources in a comparison of greenhouse gas emissions per unit of energy generated. With this translating into, from the beginning of nuclear power station commercialization in the 1970s, having prevented the emission of about 64 billion tonnes of carbon dioxide equivalent, greenhouse gases, gases that would have otherwise resulted from the burning of fossil fuels in thermal power stations.
As of 2012, according to the IAEA, worldwide there were 68 civil nuclear power reactors under construction in 15 countries, approximately 28 of which in the Peoples Republic of China (PRC), with the most recent nuclear power reactor, as of May 2013, to be connected to the electrical grid, occurring on February 17, 2013 in Hongyanhe Nuclear Power Plant in the PRC. In the USA, two new Generation III reactors are under construction at Vogtle. U.S. nuclear industry officials expect five new reactors to enter service by 2020, all at existing plants. In 2013, four aging, uncompetitive, reactors were permanently closed.
Japan’s 2011 Fukushima Daiichi nuclear disaster, which occurred in a reactor design from the 1960s, prompted a re-examination of nuclear safety and nuclear energy policy in many countries. Germany decided to close all its reactors by 2022, and Italy has banned nuclear power. Following Fukushima, in 2011 the International Energy Agency halved its estimate of additional nuclear generating capacity to be built by 2035.

