A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction of positively charged hydrogen ions with oxygen or another oxidizing agent. Fuel cells are different from batteries in that they require a continuous source of fuel and oxygen or air to sustain the chemical reaction, whereas in a battery the chemicals present in the battery react with each other to generate an electromotive force (emf). Fuel cells can produce electricity continuously for as long as these inputs are supplied.
There are many types of fuel cells, but they all consist of an anode, a cathode and an electrolyte that allow positively charged hydrogen ions (or protons) to move between the two sides of the fuel cell. The anode and cathode contain catalysts that cause the fuel to undergo oxidation reactions that generate positive hydrogen ions and electrons. The hydrogen ions are drawn through the electrolyte after the reaction. At the same time, electrons are drawn from the anode to the cathode through an external circuit, producing direct current electricity. At the cathode, hydrogen ions, electrons, and oxygen react to form water. As the main difference among fuel cell types is the electrolyte, fuel cells are classified by the type of electrolyte they use and by the difference in startup time ranging from 1 second for proton exchange membrane fuel cells (PEM fuel cells, or PEMFC) to 10 minutes for solid oxide fuel cells (SOFC). Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are “stacked”, or placed in series, to create sufficient voltage to meet an application’s requirements. In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40–60%, or up to 85% efficient in cogeneration if waste heat is captured for use.
A fuel cell combines hydrogen and oxygen to produce electricity. The basic principle of the fuel cell is illustrated in the figure below. The core of each fuel cell consists of an electrolyte and two electrodes. At the negative anode, a fuel such as hydrogen is being oxidized, while at the positive cathode, oxygen is reduced. Ions are transported through the electrolyte from one side to the other. The type of electrolyte determines the temperature window of operation. This window of operation in its turn determines the catalysts that can be used, and the purity of the fuel to be used. The theoretical open circuit voltage of a hydrogen-oxygen fuel cell is 1.23 V at 298 K, in practice it is around 1 V at open circuit. Under load conditions, the cell voltage is between 0.5 and 0.8 V.
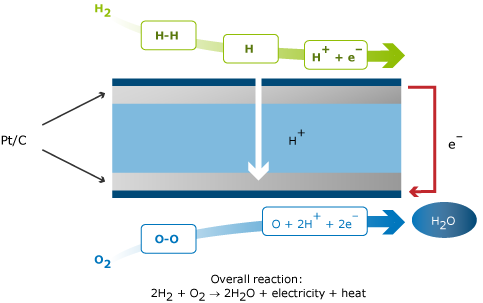
In principle, a fuel cell operates like a battery. However the cell will supply electricity as long as fuel is supplied, for example in the form of hydrogen or methanol. A fuel cell consists of two electrodes, one cathode and one anode, separated by an electrolyte. The purpose of the electrolyte is to control the spontaneous combustion of hydrogen and oxygen, known as a detonating gas explosion.
Technical Parameters
Fuel cell technology can be characterized in terms of key parameters in order to evaluate the technology. These key parameters with descriptions are presented in Table below.
| Key Parameter | Description |
| Upfront Cost | The cost of producing fuel cells in volume. |
| Operating Cost | The cost of maintaining fuel cells in the field & hydrogen supply infrastructure |
| Reliability | Reliability of fuel cells under realistic operating conditions over a period of many years. |
| Operating Temperature | Temperature under which output of fuel cell will not be affected |
| Efficiency | More efficient than internal combustion engine (ICE) |
| Emissions | Emissions of NOx, CO2, particulate or volatile organic compound emissions. |
| Sound | Noise generated during operation. |
| Smell | Odor emitted from a fuel cell during operation. |
| Catalyst | The effectiveness of the catalyst (determines reaction rates at the electrodes) |
| Fuel | Fuel options available, usually Hydrogen, except for Solid Oxide High Temperature Fuel Cell |
| Electrolyte | The type of electrolyte used in fuel cell |
| Output | Voltage given by the fuel cell |
| Energy efficiency | It is managing and restraining the growth in energy consumption. Something is more energy efficient if it delivers more services for the same energy input, or the same services for less energy input. |
Hydrogen For Fuel Cells
Hydrogen is high in energy, yet an engine that burns pure hydrogen produces almost no pollution. NASA has used liquid hydrogen since the 1970s to propel the space shuttle and other rockets into orbit. Hydrogen fuel cells power the shuttle’s electrical systems, producing a clean byproduct – pure water, which the crew drinks.
A fuel cell combines hydrogen and oxygen to produce electricity, heat, and water. Fuel cells are often compared to batteries. Both convert the energy produced by a chemical reaction into usable electric power. However, the fuel cell will produce electricity as long as fuel (hydrogen) is supplied, never losing its charge.
Fuel cells are a promising technology for use as a source of heat and electricity for buildings, and as an electrical power source for electric motors propelling vehicles. Fuel cells operate best on pure hydrogen. But fuels like natural gas, methanol, or even gasoline can be reformed to produce the hydrogen required for fuel cells. Some fuel cells even can be fueled directly with methanol, without using a reformer.
In the future, hydrogen could also join electricity as an important energy carrier. An energy carrier moves and delivers energy in a usable form to consumers. Renewable energy sources, like the sun and wind, can’t produce energy all the time. But they could, for example, produce electric energy and hydrogen, which can be stored until it’s needed. Hydrogen can also be transported (like electricity) to locations where it is needed.
Advantages and Disadvantages Of Fuel Cell Power Plants
The advantages are:
- Low emissions
- More efficient compared to a conventional internal combustion engine
- Simplicity, few if any moving parts
- Reliable and long lasting system
- Silent
The drawbacks are
- Lifetime Cost
- Hydrogen has to be produced
- Not yet available infrastructure for hydrogen

